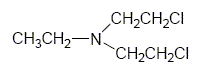| ||||||||||||||||||||||||||||||||||||||||||||||
This group of agents includes the sulfur mustards (H and HD) and the nitrogen mustards (HN-1, HN-2, and HN-3). Because of their physical properties, mustards are persistent under cool conditions however, evaporation increases as the temperature increases. [Zajtchuk] It is possible to increase their persistency even more by dissolving them in thickeners.[NATO] Mustard agents are a oily liquid with a weak, sweet, agreeable odor; are sparingly soluble in water and soluble in fat, fat solvents, and other common organic solvents. Mustards volatilizes in steam; are combustible when exposed to heat or flame. When heated to decomposition, emits very toxic fumes of sulfur oxides (SOx), hydrochloric acid, and other chlorinated compounds. There is sufficient evidence for the carcinogenicity of mustard gas in humans. Several studies have shown an increased mortality from respiratory tract cancer among individuals exposed to mustard gas. This mortality was greater in those individuals with long-term occupational exposure than in those with sporadic exposure. Mustard gas is used primarily as a model compound in biological studies of alkylating agents. Researchers have tested mustard gas as an antineoplastic agent, but its clinical use as a tumor inhibitor has been minimal. Use of mustard gas in chemical warfare occurred mainly during World War I. [ATSDR 1998] Mustards are not found naturally in the environment in any amount; therefore, there are no background levels in the soil, air, water. Currently, all of the mustards at Army bases are being destroyed by burning or neutralization. If mustarda are put on soil, it will remain there for at least a day, but may remain for several days or longer. The time it takes for mustard to disappear from soil depends on how hot it is outside and how strongly the wind is blowing. If it is hot and the wind is strong, then mustard will disappear faster. When mustard disappears from soil, it becomes a vapor or changes into other compounds if the soil is wet. If mustard is buried underground, it may not disappear for several years. Mustard will not move through soil to underground water. If mustard is put in water, it dissolves within minutes if the water is stirred, and slowly if is not. When it does dissolve, it reacts with water and changes to other compounds. The time necessary for a quantity of mustard that is dissolved in water to decrease by half is about 2 minutes at 40 °C. If large amounts of mustard are spilled into water, most of the mustard will change to other compounds very slowly or not at all. If mustard is released into air, it will react with components in the air to form other compounds. The time necessary for a quantity of mustard in air to decrease by half is about 2 days at 25 °C. Because mustard changes to other chemicals in the environment, it will not concentrate in plants or animals. The general public may be exposed to mustard at hazardous waste sites that contain mustard. In addition, the use of mustard by terrorists is of concern. Persons involved in the transport or disposal of mustard may be exposed to mustard agents generated unintentionally through mishap. Spouses, children, and others may be exposed if workers unknowingly bring the mustard agents out of the factory on their skin or clothing. Mustard readily passes through ordinary clothing. Mixed in water, mustard changes its form within minutes, so it is very unlikely that you would drink it. The likelihood of the general population being exposed by way of water (drinking, cooking, bathing, and swimming) is therefore very small. [ATSDR 2003]
|
||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||
|
Sulfur mustard was first manufactured in 1822. It was utilized as early as the late 1880s, when it was used as a pesticide and to treat minor tumors. It was first used as a war gas in 1917, during World War I by the Germans on the British at Ypres. For this reason, sulfur mustard is also called yperite. Sulfur mustard is a component of the H-series blister agents including undistilled sulfur mustard (H; sulfur mustard with 20–30% impurities, also known as Levinstein mustard), distilled sulfur mustard (HD or HS; approximately 96% pure), a mustard-lewisite mixture (HL), an HD/agent T mixture (HT; a mixture of HD and nonvolatile agent T), and an HD/agent Q mixture (HQ; a mixture of HD and nonvolatile agent Q; agent Q is also known as sesqui-mustard). Distilled Mustard (HD) is a pale yellow to dark brown oily liquid with a garlic-like odor. [Abercrombie] Levinstein Mustard (H) is the original mustard (gas) of World War I vintage. Properties of H are essentially the same as those for HD. [USACHPPM]
|
||||||||||||||||||||||||||||||||||||||||||||||
|
Structural Formula:
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||
|
Nitrogen Mustard (HN-1) is a colorless liquid when pure with a faint, fishy or soapy odor. [DoD 2000]
|
||||||||||||||||||||||||||||||||||||||||||||||
|
Structural Formula:
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||
|
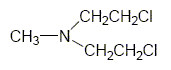
Nitrogen Mustard (HN-2) is a colorless liquid when pure with a faint, fishy or soapy odor. [DoD 2000]
|
||||||||||||||||||||||||||||||||||||||||||||||
|
Structural Formula:
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||
|
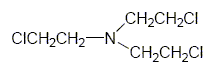
Nitrogen Mustard (HN-3) is a colorless, odorless liquid when pure. [Abercrombie] It is the most stable in storage of the three nitrogen mustards. [Kirner]
|
||||||||||||||||||||||||||||||||||||||||||||||
|
Structural Formula:
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||
Toxicology |
||||||||||||||||||||||||||||||||||||||||||||||
|
Mustards are chemicals that cause tissue blistering. Their toxic activity is, however, not limited to the skin and their mode of action is complex. These cytotoxic alkylating agents were initially developed as chemical weapons used to induce ocular, dermal, and respiratory damage resulting in immediate casualties, reduction in fighting efficiency, and demoralization. Depending on the exposure, injury may be local or systemic. Although nitrogen mustards were specifically developed as military agents, HN-1 was originally developed as a pharmaceutical. HN-2 (mechlorethamine) later found use as an antineoplastic agent. Derivatives such as melphalan, chlorambucil, and cyclophosphamide are alkylating agents used as cancer therapeutic agents [Somani, 1992]. Mustards when sprayed onto soil, a vesicant action may persist for about 2 weeks but when the agent continually leaks into the soil vesicant action may be present after 3 years [DoD 1974). Persistence of sulfur mustard in soil is due to the formation of oligomeric degradation products that coat the surface of the mustard agent and that are resistant to hydrolysis [Rosenblatt 1995]. This may greatly enhance the environmental persistence of sulfur mustard. Sulfur mustard has a log Kow of 1.37 and a Koc of 133, indicating that binding to soil organics would limit transport through soil to groundwater. Mustard has a leaching index 7.2 (i.e. the number of leachings required to reduce the HD soil concentration to one-tenth of the original amount, assuming that for each leaching one kilogram of soil is in equilibrium with one liter of water). [MacNaughton 1994] |
||||||||||||||||||||||||||||||||||||||||||||||
Toxicokinetics With its high lipophilicity, toxicologically relevant amounts of sulfur mustard are absorbed into epithelial tissue [Papirmeister 1991]. Dermal absorption is dependent on the thickness of the epidermis and on the presence of moisture, which enhances penetration. Absorption tends to be greater at the base of hair shafts and in the hair follicle where the epithelial tissue is thinner than the surrounding surface area [Papirmeister 1991]. Approximately 20% of sulfur mustard applied to skin may be rapidly absorbed while 12–50% of this may react and remain at the application site [Somani 1989]. About 12% of that absorbed remains at the contact site and the remaining 88% enters the circulation, rate of penetration is 1–4 mg/cm2/min at 75oF.[Renshaw 1947]. The effects of time, temperature, and humidity on the vapor penetration of HN-1 and HN-3 into the forearm skin of human male volunteers were reported. [NDRC 1945]. Results of this work showed similar effects of temperature and humidity as observed for sulfur mustard, e.g. greater absorption with increased temperature and humidity. The penetration of HN-1 and HN-3 was found to be linear with time (5 to 20 min for HN-1 and 30–60 min for HN-3). At 71–72oF and 50–52% relative humidity, HN-1 penetration rate was 2.8 mg/cm2/min and for HN-3 was 0.18 mg/cm2/min at 72–73oF and 45–48% relative humidity. At 86–87oF and 47–49% relative humidity, HN-1 penetration rate increased to 5.2 mg/cm2/min and HN-3 penetration rate increased to 0.3 mg/cm2/min at 85oF and 47–48% relative humidity. Several studies using radiolabeled sulfur mustard have shown that sulfur mustard and its metabolites may be widely distributed in the body after percutaneous or intravenous exposure. Maximum levels of radioactivity were detected in the kidney, lungs, and liver of rabbits following intravenous administration [Boursnell 1946]. At 15 min following percutaneous exposure of rats, sulfur mustard derived radioactivity was found in all examined tissues except the eyes [Young 1944]. Uniform distribution of radioactivity in mice after either percutaneous or intravenous exposures, with most radioactivity occurring in the nasal region, kidneys, liver, and intestine. [Clemedson 1963] Much of the agent entering the blood binds to hemogloblin and, to some extent, with glutathione. [Hambrook 1993] Results of studies with rabbits showed that sulfur mustard concentrated in the cornea and to a lesser extent in the iris, lens, and conjunctiva within 5 min after application. [Axelrod 1947] The biotransformation of sulfur mustard after intravenous or intraperitoneal injection of radiolabeled compound in rats has been examined. Following intravenous injection, the major urinary metabolite was glutathione-bischloroethyl sulfide conjugates (45% of total urinary radioactivity) and smaller amounts of sulfone conjugates (7%) and thiodiglycol and its conjugates (14.4%). [Davison 1961] The major urinary product of cysteine-bis-(b-chloroethyl)sulfone after intraperitoneal injection of sulfur mustard in rats. [Roberts 1963] The hydrolysis to thiodiglycol and reaction with glutathione are the most important routes of detoxification. [Papirmeister 1991] This is supported by human data showing that thiodiglycol is present in the urine for one week or more after exposure. [Wils 1987] Excretion via the urine is likely a major route of elimination.
The mechanism of action of mustards is multifaceted and complex. [Papirmeister 1991] [Hurst 2008] Efforts to understand the mechanisms of sulfur mustard toxicity are ongoing. Basically sulfur mustard disrupts the interface of the epidermis and basement membrane causing blistering between the epidermis and dermis. Both immediate (immediate cell membrane damage) and delayed phases (secondary effects resulting from inflammatory responses, DNA damage, vascular leakage) have been described for sulfur mustard-induced dermal effects. [Somani 1989] Many of the toxic effects of sulfur mustard can be attributed to oxidative stress. Among the most studied mechanisms of sulfur mustard toxicity are thiol depletion resulting in intracellular calcium imbalance and subsequent cell death, alkylation of DNA and other cellular macromolecules, lipid peroxidation resulting from sulfur mustard-induced gluthathione depletion, and induction of an inflammatory response. The overall mechanism of sulfur mustard toxicity likely involves an interlinking of the aforementioned processes. A key component of sulfur mustard toxicity is the formation of a sulfonium ion and resulting episulfonium intermediate which may react with sulfhydryl-containing macromolecules. Damage may include Ca2+ translocases (Ca2+-stimulated, Mg2+-dependent ATPase) which are dependent on thiol groups to maintain cellular Ca2+ homeostasis, and microfilamentous proteins. The resulting increase in intracellular calcium levels ultimately causes a decrease in cellular integrity and induction of apoptosis. Oxidative stress in sulfur mustard toxicity has been reviewed. [Smith 2008] The role of DNA alkylation and the poly(ADP-ribose)polymerase (PARP) hypothesis theory for sulfur mustard toxicity has been reviewed [Papirmeister 1991]. In this mechanism, DNA is the initial target of the mustard agent. Alkylated DNA purines are enzymatically depurinated creating apurinic sites which are cleaved by apurinic endonucleases resulting in DNA strand breaks. The accumulation of DNA breaks leads to activation of the chromosomal enzyme PARP, which utilizes NAD+ causing severe lowering of cellular NAD+. Depletion of NAD+ results in the inhibition of glycolysis, and stimulation of the nicotinamide adenine dinucleotide phosphate (NADP+)-dependent hexose monophosphate shunt, ultimately resulting in the induction and secretion of proteases and subsequent cellular changes. Sulfur mustard-induced cytotoxicity is dose dependent [Papirmeister 1991] and DNA appeared to be more sensitive to mustard-induced alkylation than are other cellular constituents. The low-dose effects of sulfur mustard are characterized by genotoxicity and inhibition of mitosis. The loss of cellular reproduction may be due to bifunctional alkylation that ultimately prevents normal DNA replication. It was hypothesized that monofunctional DNA damage might be responsible for low-dose mutagenic and possibly carcinogenic effects. Sulfur mustard-induced lipid peroxidation is a function of glutathione (GSH) depletion. For this mechanism, depletion of GSH results in an accumulation of reactive oxygen species via hydrogen peroxide-dependent processes. [Miccadei 1988] The oxygen radicals react with membrane phospholipids forming lipid peroxides that alter membrane structure resulting in membrane breakdown. Recent work has focused on the identification of possible biomarkers of sulfur mustard exposure and injury [Buxton 2000, 2001] [Danne 2000]. More recently, the role of metalloproteinases and collagen degradation [Gerecke 2005], platelet activating factor [Clark 2005, 2006], and interaction with cytochrome P450 processes [Brimfield 2005, 2006] [Mancheco 2006] are being investigated relative to the mechanism of action of sulfur mustard. A key component of nitrogen mustard toxicity is analogous to that of sulfur mustard: the formation of a cyclic onium cation. This occurs in the presence of polar solvents such as water [Somani 1992]. The immonium ion may react with nucleophiles such as nitrogen in the base components of nucleic acids and with sulfhydryl groups in proteins and peptides. The precise mechanism of nitrogen mustard activity is unclear but several have been proposed: DNA/RNA alkylation and resultant effects, effects on glutathione, membrane effects (protein cross-linking, ion transport effects), and cytoplasmic effects (release of lysosomal enzymes). The possible mechanisms of nitrogen mustard have been reviewed. [Gray 1989] In mice given HN-2 intraperitoneally showed pulmonary alterations indicative of oxidative stress and impaired detoxification processes which are consistent with the aforementioned mechanisms. [Elsayed 2006] |
||||||||||||||||||||||||||||||||||||||||||||||
The toxic effects of sulfur mustard in humans and animals have been extensively reviewed. [ATSDR 2003] [Sidell 1992] [Somani 1992] [Watson 1992] [IOM 1993] [NRC 2003] [Romano 2008] Mustards affects the skin, respiratory tract, and eyes. The acute effects include edema, ulceration, and necrosis of epithelial tissue. Systemic toxicity may also occur and is characterized by nausea and vomiting, fever, and malaise. There is evidence of systemic toxicity (gastrointestinal tract) following dermal exposure only. [Dacre 1996] Delayed effects include conjunctivitis and blindness following ocular exposure and chronic bronchitis following inhalation exposure. Affected tissues may have an increased susceptibility to secondary infections, and possibility of carcinogenicity of the skin and respiratory tract. Ambient temperature and humidity govern the degree of toxicity of mustards; in hot and humid conditions, lower mustard concentrations are required to produce debilitating effects. The severity of mustard effects is also greater in areas of the body with greater moisture (e.g. axilla, groin, eyes). Information regarding the toxic effects of long-term exposure to low levels of sulfur mustard that are not acutely toxic is limited. Available data suggest that the location and severity of damage resulting from exposure to sulfur mustard are concentration dependent and a function of the highly reactive nature of sulfur mustard. [Papirmeister 1991] The eyes are generally considered to be the most sensitive and rapidly responding target. [Reed 1918, 1920] [Anderson 1942] For low exposures, sulfur mustard-induced injury appears to be limited to the upper respiratory tract [Eisenmenger 1991] and eyes [Reed 1918, 1920] [Guild 1941] [Anderson 1942] In work with informed volunteer subjects, Anderson [Anderson 1942] reported that Ct values of 60–75 mg-min/m3 would result in conjunctivitis, photophobia, and ocular irritation, while Ct values of 75–90 mg-min/m3 would cause a high proportion of casualties as determined by more severe ocular damage requiring several weeks of treatment. At higher concentrations, pulmonary effects would be expected. [Eisenmenger 1991] Regardless of the target tissue, there is a latency period between initial exposure and development of effects. The eyes and respiratory tract appear to have the shortest latency period with effects appearing within hours depending on the exposure level. In addition to the acute toxic effects on the eyes, skin, and respiratory tract, both acute and longer-term neuropsychiatric effects (e.g. depression, anxiety, neurasthenia, insomnia, post-traumatic stress syndrome) have been documented for individuals exposed to sulfur mustard. [Romano 2008] Many of these effects have been documented for individuals exposed during noncombat (e.g. munitions plant workers) activities and are not always the result of high-level exposure that result in serious overt effects. Longer-term effects such as chronic bronchitis have been associated with occupational exposures that included episodes of acute toxicity, and delayed or recurrent keratitis may occur 8–40 years after a severe vapor exposure. Sulfur mustard-induced immunosuppression resulting in greater susceptibility to infections has also been reported. Acute lethality data in animals are summarized in Table. Based upon the animal data, interspecies variability in the lethal response to sulfur mustard vapor is less than an order of magnitude. For nonlethal effects, the animal data suggest that test species exhibit signs of toxicity that are qualitatively similar to humans when acutely exposed to sulfur mustard vapor. Ocular and respiratory tract irritations are clearly evident in studies using dogs, rats, mice, rabbits, and guinea pigs. TABLE
Effects of orally administered sulfur mustard in rats were studied by [Sasser 1989]. Repeated gavage administration of sulfur mustard in sesame oil produced epithelial hyperplasia of the forestomach at the highest dose tested but no deaths and no other treatment-related pathological lesions or changes in clinical chemistry or hematological parameters. Results of a multigeneration study in rats given sulfur mustard by gavage showed no significant adverse effects on reproductive parameters at any dose level, but revealed dose-related lesions of the squamous epithelium of the forestomach (acanthosis and hyperplasia). It is likely that the forestomach lesions were a function of the treatment regimen whereby the bolus dose in an oil vehicle (sesame seed oil) would enhance the direct-contact effects of the sulfur mustard on the forestomach tissue. Studies [Hackett 1987] in which rabbits were gavage dosed with sulfur mustard were equivocal regarding reproductive/developmental effects due in part to the dose regimen and overt maternal toxicity. Studies in animals have shown that sulfur mustard may induce developmental and reproductive effects [NRC 1999, 2003]. Acute exposures resulting in systemic uptake may have effects on reproductive organs, including inhibition of spermatogenesis. Fetal anomalies were observed in tests with rats given sulfur mustard during gestation but only at maternally toxic doses. The genotoxicity of sulfur mustard is well documented. It is known to produce DNA cross-links, mutations following replication or repair errors, chromosomal breaks, and chromosomal aberrations. Occupational exposures have been associated with increased frequencies of somatic cell mutations, sister chromatid exchanges, and chromosome abnormalities. Studies with rats indicate that subchronic inhalation or oral exposures can produce dominant lethal effects. The carcinogenicity of sulfur mustard in animals has been reviewed. [IARC 1975] [Watson 1989] [IOM 1993] [NRC 1999] [USACHPPM 2000] An increased incidence of pulmonary tumors in Strain A mice was observed following intravenous injections (four doses over 2 days) of sulfur mustard [Heston 1950] and an increase in injection site tumors in mice given subcutaneous injections of sulfur mustard over a 6-week period. [Heston 1953] A study of the Iranian military veterans exposed to sulfur mustard under battlefield conditions during the Iran–Iraq conflict at levels sufficient to cause severe signs of toxicity indicated a potential increased incidence of chronic myelocytic leukemia (CML). In several earlier studies on WWI veterans who had been exposed to sulfur mustard, leukemia was not identified as a possible effect, although it is unclear if examination for CML had ever occurred in those populations. Confounders, such as exposure to benzene or radiation which complicate the analysis, have not yet been ruled out in the ongoing epidemiologic study of Iranian veterans. Two cases of CML were reported for Japanese workers exposed to sulfur mustard [Shakil 1993] but the incidences of CML in the entire population of sulfur mustard-exposed workers and in an unexposed control population were not reported. Studies in animals provide supporting evidence for the carcinogenicity of sulfur mustard although the results of some studies are compromised by insufficient exposure durations and injuries resulting from caging situations. Information regarding the toxicity of nitrogen mustards is not as extensive as that for sulfur mustard. Like sulfur mustard, exposure to nitrogen mustards may cause skin blistering as well as respiratory tract injury and ocular damage. Response data from tests with informed human volunteer subjects [NDRC 1944] suggested a relative potency of HN-3 > HN-1 > HN-2 for vesicant effects, although the differences were minor. Like sulfur mustard, dermal effects were enhanced by moisture (as from sweating). Ocular injury (irritation resulting in compromised operational effectiveness of military personnel) was detected at exposures much lower than those causing dermal effects. All of the toxic effects of nitrogen mustard appear to involve a latency period of several hours for ocular responses and several days for dermal blistering. Nitrogen mustards are alkylating agents with known mutagenicity, but there are no animal cancer bioassays and no human carcinogenicity data. Nitrogen mustard and its hydrochloride salt have been shown to be teratogenic in mice and rats. Intraperitoneal administration of HN-2-hydrochloride to mice during gestation resulted in serious teratogenic effects. [Danforth 1954] [Haskin 1948] [Murphy 1958] Nitrogen mustards are bifunctional alkylating agents that produce a carcinogenic response (primarily lung tumors and lymphomas) in mice following subcutaneous, intraperitoneal, and intravenous administration as well as by skin painting. [IARC 1987a] Intravenous administration of nitrogen mustard to rats produced tumors in multiple organs [IARC 1987b] Information in humans is limited to reports of squamous cell carcinomas of the skin following therapeutic application of nitrogen mustard in the treatment of mycosis, fungoides, and psoriasis. |
||||||||||||||||||||||||||||||||||||||||||||||
Various standards and guidelines have been developed for mustards. These values are applicable to occupational exposures, emergency planning and response efforts, and remediation efforts. Airborne exposure limits (AELs) and health-based environmental screening levels (HBESLs) for sulfur mustard have been developed by the US Army. [USACHPPM 1999, 2000] Most health-based criteria for sulfur mustard vapor exposure are based upon protection of the eyes and respiratory tract which are the most sensitive targets. Acute Exposure Guideline Levels (AEGLs) for sulfur mustard have been developed for emergency planning and emergency response applications. [NRC 2003] The AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 min to 8 h. Reference doses (RfDs), an estimate of a daily dose to humans that is likely to be without appreciable risk of deleterious health effects during a lifetime, have also been developed for sulfur mustard. [NRC 1999] The various guidelines and standards for sulfur mustard have been summarized. [ATSDR 2003] Very few standards and guidelines are available for nitrogen mustards. AEGL values for the nitrogen mustards, HN-1, HN-2, and HN-3, have been developed and are based upon ocular irritation in human volunteers (AEGL-2) and lethality in rodents (AEGL-3). Data were insufficient for derivation of level AEGL-1 values. The AEGL values are currently awaiting finalization. The US Army has developed Worker Population Limit (WPL) values and General Population Limit (GPL) values for nitrogen mustard [USACHPPM 1996, 2004] The International Agency for Research on Cancer (IARC) classified sulfur mustard as a Group 1 carcinogen (carcinogenic to humans). [IARC 1987] The National Toxicology Program (NTP) considers ‘‘mustard gas’’ as a substance ‘‘known to be a human carcinogen’’. [DHHS 2002]. These assessments are based upon human and animal data. Studies of occupational exposures to sulfur mustard indicate an elevated risk of respiratory tract and skin tumors following long-term exposure to acutely toxic concentrations. Overall, several factors are important regarding the assessment of the carcinogenicity of sulfur mustard. Increased cancer incidence in humans appears to be associated only with exposures that caused severe acute effects, and occupational exposures tended to involve repeated exposures and repeated injury of the same tissues. Because the therapeutic use of the sulfur mustard analog nitrogen mustard is associated with an increased incidence of CML, the reports of CML in HD-exposed individuals appear to be relevant to the carcinogenicity of sulfur mustard. Cancer slope factors and unit risk values for sulfur mustard have been summarized. [ATSDR 2003] Data are not available with which to quantitatively assess the cancer risk from nitrogen mustards, although [IARC 1987] considers nitrogen mustard a Group 2A carcinogen based upon limited evidence in humans and sufficient evidence in animals. |
||||||||||||||||||||||||||||||||||||||||||||||
Medical management of mustards exposure begins with prevention of exposure, the military use of sulfur mustard necessitated full-body protection. As a result, considerable effort has been expended in the development and evaluation of protective clothing and equipment. [Schier 2005] In general, these include respirators (air-purifying and atmosphere-supplying), and chemical-protective clothing (e.g. chemical and vapor impermeable coverings, clothing treated with adsorbing or neutralizing chemicals). Following exposure, rapid decontamination is essential and may include removal of contaminated clothing and removal/neutralization of the agent. Ocular exposure will necessitate rapid removal of the agent from the eyes by irrigating with water. Vapor exposure may necessitate respiratory support. Because there are no antidotes for sulfur mustard poisoning, medical management must rely on prevention, decontamination, and palliative treatment of signs and symptoms. The use of possible antidotes (e.g. antioxidants) has been reviewed [Smith 2008] Polyurethane sponges containing detoxification additives are currently being developed and evaluated for decontamination/detoxification. [Gordon 2006] The medical management of sulfur mustard (and other vesicant agents) has been reviewed. [Munro 1990] [Keyes 2005] Medical management of nitrogen mustard exposure is similar to that for sulfur mustard and involves prevention of exposure and, where exposure has occurred, decontamination and support therapy. The use of antioxidants in the treatment of nitrogen mustard toxicity is currently under investigation. [Hardej 2006] |
||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||
references |
||||||||||||||||||||||||||||||||||||||||||||||
|
Abercrombie, P. L., and Butrow, A.B.,
Selected Physical Properties of Ton Container HD (Mustard) and VX,
ERDEC-TR-450, U.S. Army Edgewood Research, Development, and Engineering
Center, Aberdeen Proving Ground, MD, July 1998, UNCLASSIFIED Report
(ADA350462). Anderson, J.S. 1942. The Effect of Mustard Gas Vapour on Eyes under Indian Hot Weather Conditions. CDRE Report No. 241. Chemical Defense Research Establishment (India). ATSDR, KNOWN TO BE A HUMAN CARCINOGEN: Mustard gas, CAS No. 505-60-2. Agency for Toxic Substances and Disease Registry, 1998, 1 p. ATSDR, TOXICOLOGICAL PROFILE FOR SULFUR MUSTARD. Agency for Toxic Substances and Disease Registry, 2003, 217pp.217 Axelrod, D.J. and J.G. Hamilton. 1947. Radio-autographic studies of the distribution of lewisite and mustard gas in skin and eye tissues. Am. J. Pathol. 23:389-411. Bartlett, P.D., and Swain, C.G., “Kinetics of Hydrolyisis and Displacement Reactions of ß ß’-(Dichlorodiethyl Sulfide (Mustard Gas) and of ß -Chloro- ß’-hyroxidediethyl Sulfide (Mustard Chlorohydrin),” J. Chem. Soc., Vol. 71, 1949. Boursnell, J.C., J.A. Cohen, M. Dixen, et al. 1946. Studies on mustard gas (ßß' -dichlorodiethyl sulphide) and some related compounds. 5. The fate of injected mustard gas (containing radioactive sulphur) in the animal body. Biochem. J. 40:756-764. Bowden, E. (1943). Median detectable concentrations by odor of plant run mustard, plant run Lewisite and pilot plant ethyl nitrogen mustard. ADB 969801, TDMR 615 (April). Brimfield, A.A., Hodgson, E. (2005). Observations on the interaction of sulfur mustard with cytochrome P450. Toxicologist 84(S-1): 159. Brimfield, A.A., Novak, M.J., Mancebo, A.M., Gallagher, B.S., Arroyo, M. (2006). The detection of free radical formation from the interaction of sulfur mustard with NADPHcytochrome P450 reductase. Toxicologist 90: 391. Brookfield, K. J.; Woodward, F. N.; Owens, R. The kinetics of hydrolysis of vesicants. Part II. 2,2′-dichlorodiethylsulphide (H). Sutton Oak Report 576; Military Intelligence Division: Sutton Oak, U.K., Mar 3, 1942. Brooks, M. E. and Parker, G.A., Incineration/Pyrolysis of Several Agents and Related Chemical Materials Contained in Identification Sets, ARCSL-TR-79040, October 1979, UNCLASSIFIED Report (ADB042888). Buckles, M.F., CW Vesicants: Selected Values for the Physical Properties of H, T, and Q (U), Special Report CRLR 542, Chemical Corps Chemical and Radiological Laboratories, Army Chemical Center, MD, May 1956, UNCLASSIFIED Report (AD108272). Buxton, K.L., Babin, M.C., Ricketts, K.M., Gazaway, M.Y., Blank, J.A., Danne, M.M. (2000). Characterization of sulfur mustard-induced proinflammatory mediator response in mouse ears. Toxicologist 54: 213. Buxton, K.L., Danne, M.M., Babin, M.C., Ricketts, K.M., Gazaway, M.Y., Sabourin, C.L., Casillas, R.P., Schlager, J.J. (2001). Gene array analysis of sulfur mustard-induced inflammatory mediator response in mouse ear. Toxicologist 60: 129. Cheicante, R.L., et al., “Investigation for the Determination of Nitrogen Mustard and Related Compounds in Air by Gas Chromatography Using Solid Sorbent Collection and Thermal Desorption,” In Proceedings of the 1998 ERDEC Scientific Conference on Chemical and Biological Defense Research 17-20 November 1998, UNCLASSFIED Paper, ERDEC-SP-004, USA Edgewood Chemical Biological Center, Aberdeen Proving Ground, MD, July 1999, UNCLASSFIED Report (ADA375171). Clark, O.E., Neally, E.W., Leiter, K., Miller, A.L., Nicholson, J.D., Smith, W.J. (2006). Endothelial cell alterations following in vitro exposure to sulfur mustard or platelet activating factor. Toxicologist 90: 391. Clark, O.E., Neally, E.W., Leiter, K.W., Finke, K.I., Miller, A.L., Smith, W.J. (2005). Putative role of platelet activating factor (PAF) analogs in cell cycle aberrations in human endothelial cells in vitro. Toxicologist 84(S-1): 160. Clemedson, C.-J., H. Kristoffersson, B. Sorbo and S. Ullberg. 1963. Whole body autoradiographic studies of the distribution of sulphur 35-labelled mustard gas in mice. Acta Radiol. Ther. 1:314-320. Dacre JC, and Goldman M, “Toxicology and Pharmacology of the Chemical Warfare Agent Sulfur Mustard,” Pharmacol Rev, 48, 1996, pp. 289–326. Danforth, C.H., Center, E. (1954). Nitrogen mustard as a teratogenic agent in the mouse. Proc. Soc. Exp. Biol. Med. 86: 705–7. Danne, M.M., Babin, M.C., Gazaway, M.Y., Ricketts, K.M., Schlager, J.J., Buxton, K.L. (2000). Gene expression array analysis of sulfur mustard-induced proinflammatory mediator response in mouse ears. Toxicologist 54: 213. Davison, C., R.S. Rozman and P.K. Smith. 1961. Metabolism of bis-ßchloroethyl sulfide (sulfur mustard gas). Biochem. Pharmacol. 7:65-74. Dawson, T.P., and Witten, B., Bis (2-chloroethyl) methylamine, Preparation, Decontamination, and Stability, Technical Division Memorandum Report 442, Chemical Warfare Center, Edgewood Arsenal, MD, September 1942, UNCLASSIFIED Report (ADB960331). Dawson, T., and Witten, B., A Memorandum Report New Compounds 2,2’ Dichlorotriethylamine, Technical Division Memorandum Report 552, USA Chemical Research and Development Laboratories, Army Chemical Center, MD, February 1943, UNCLASSFIED Report (ADB960467). DHHS, Report on Carcinogens, Eleventh Edition, U.S. Department of Health and Human Services, 2002, pp. 706 DoD TM 3-215/AFM 355-7, Military Chemistry and Chemical Agents, December 1963, UNCLASSIFIED Technical Manual (ADA292141). DoD Chemical Agent Data Sheets, Vol. 1. Tech Rpt EO-SR-74001 (Edgewood Arsenal Special Report), AD B028222. Aberdeen Proving Ground, MD:U.S.Department of the Army, Edgewood Arsenal, 1974;167-179. DoD, NBC DECONTAMINATION, U.S. Department of Defense, 2000, pp. 229 Dudley, H.C., Wells, W.J.H.B. (1938). The detection of HS by odor. ADB 959500, EATR 249 Eisenmenger, W., Drasch, G., von Clarmann, M., Kretschmer, E., Roider, G. (1991). Clinical and morphological findings on mustard gas [bis(2-chloroethyl)sulfide] poisoning. J. Forensic Sci. 36: 1688–98. Elsayed, N., Omaye, S. (2006). Pulmonary biochemical alterations induced by systemic administration of nitrogen mustard. Toxicologist 90: 392. Felsing, W.A., et al., “The Melting Point of Mustard Gas,” J. Amer. Chem. Soc., Vol. 70, 1948. Franke, S., Manual of Military Chemistry Volume I- Chemistry of Chemical Warfare Agents, ACSI-J-3890, Chemie der Kampfstoffe, East Berlin, April 1968, UNCLASSIFIED Technical Manual (AD849866). Fuhr, I., Krakow, E.H. (1945). Median lethal concentrations of H for mice and rats. For various exposure times. MDR 21, March 21 Gerecke, D.R., Bhatt, P., Chang, Y. et. al. (2005). The matrix metalloproteinase inhibitor GM 1489 reduces MMp-9 activity after sulfur mustard exposure in vivo. Toxicologist 84 (S-1): 451. Gordon, R., Baker, K., Askins, L., Ratcliffe, R., Lindsay, D., Owens, R. et al. (2006). Chemical warfare agents (organophosphates and vesicant) and biological decontamination and detoxification using polyurethane sponges. Toxicologist 90: 393. Gray, P.J. (1989). A literature review on the mechanism of action of sulphur and nitrogen mustard. MRL Tech. Report MRLTR-89-24. DSTO Materials Research Laboratory, Victoria, Australia. Guild, W.J.F., K.P. Harrison, A.Fairley and A.E. Childs. 1941. The Effect of Sulfur Mustard on the Eyes. Porton Report 2297. Chemical Defense Experimental Station, Porton, UK. Hackett, P.L., R.L. Rommereim, F.G. Burton, R.L. Buschbom and L.B. Sasser. 1987. Teratology Studies on Lewisite and Sulfur Mustard Agents: Effects of Sulfur Mustard in Rats and Rabbits. Final Report. AD A187495. Pacific Northwest Laboratory, Richland, WA. Prepared for the U.S. Army Medical Research and Development Command, Fort Detrick, MD. Hambrook, J.L., D.J. Howells and C. Schock. 1993. Biological fate of sulphur mustard (1,1'-thiobis(2-chloroethane)): uptake, distribution and retention of 35S in skin and in blood after cutaneous application of 35S-sulphur mustard in rat and comparison with human blood in vitro. Xenobiotica 23:537-561. Hardej, D., Billack, B. (2006). Reduction of mechlorethamine cytotoxicity by ebselen in normal and tumor-derived cell lines. Toxicologist 90: 391 Harris, B.L., et al., Thickened Vesicants: Storage Stability of Unthickened and Thickened Nitrogen Mustards and Their Mixtures with Levinstein Mustard, Technical Division Memorandum Report 706, USA Chemical Research and Development Laboratories, Army Chemical Center, MD, July 1943, UNCLASSIFIED Report (ADB962153). Harris, B.L. and Macy, R., Corrosion by Vesicants: Rate of Corrosion of Steel and Other Metals by H, HQ, HN-3, HN-1, and L, Mostly at 65°C, Technical Division Memorandum Report 1031, USA Chemical Research and Development Laboratories, Army Chemical Center, MD, April 1945, UNCLASSIFIED Report (ADB963161). Haskin, D. (1948). Some effects of nitrogen mustards on the development of the external body form in the fetal rat. Anat. Rec. 102: 493–511. Heston WE. Induction of pulmonary tumors in strain A mice with methyl-bis (beta-chloroethyl)amine hydrochloride. J Natl Cancer Inst. 1949;10:125–130. Heston, W.E. 1950. Carcinogenic action of mustards. J. Natl. Cancer Inst. 11:415-423. Heston, W.E. 1953. Occurrence of tumors in mice injected subcutaneously with sulfur mustard and nitrogen mustard. J. Natl. Cancer Inst. 14:131-140. Heston, W.E. and W.D. Levillain. 1953. Pulmonary tumors in strain A mice exposed to mustard gas. Proc. Soc. Exp. Biol. 82:457-460. Hurst, C.G., Smith, W.J. (2008). Health effects of exposure to vesicant agents. In Chemical Warfare Agents, Chemistry, Pharmacology, Toxicology, and Therapeutics (J. Romano, Jr., B. Lukey, H. Salem, eds), pp. 293–312. CRC Press, Boca Raton, FL. IARC (International Agency for Research on Cancer). 1975. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man: Some Aziridines, N, S, & O-mustards and Selenium, vol. 9, pp. 181-207. International Agency for Research on Cancer, Lyons, France. IARC (International Agency for Research on Cancer). 1987a. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man: Genetic and Related Effects: An Updating of Selected IARC Monographs Volumes 1-42,Suppl. 6,International Agency for Research on Cancer, Lyons,France. IARC (International Agency for Research on Cancer). 1987b. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man: Overall Evaluation of Carcinogenicity: An Updating of IOM (Institute of Medicine, Committee to Survey the Health Effects of Mustard Gas and Lewisite, Division of Health Promotion and Disease Prevention). 1993. Veterans at Risk; The Health Effects of Mustard Gas and Lewisite, C.M. Pechura and D.P. Rall, eds. National Academy Press, Washington,DC. Keyes, D.C., Burstein, J.L., Schwartz, R.B., Swienton, R.E. (eds) (2005). Medical Response to Terrorism. Lippincott Williams & Wilkins, Philadelphia, PA. Kibler, A.L., Data on Chemical Warfare, Technical Division Memorandum Report 456, Chemical Warfare Center, Edgewood Arsenal, MD, November 1942, UNCLASSIFIED Report (ADB969725) Kirner W.R., Summary Technical Report of Division 9, NDRC Volume 1, Chemical Warfare Agents, and Related Chemical Problems Part I-II, Office of Scientific Research and Development, Washington, DC, 1946, UNCLASSIFIED Report (AD234270). MacNaughton, M. G.; Brewer, J. H. EnVironmental Chemistry and Fate of Chemical Warfare Agents;Southwest Research Institute: San Antonio TX, 1994. Mancheco, A.M., Brimfield, A.A. (2006). Interaction of sulfur mustard with NADPH-cytochrome P450 reductase and cytochrome P450 isoforms. Toxicologist 90: 391. Miccadei, S., Kyle, M.E., Gilfor, D., Farber, J.L. (1988). Toxic consequences of the abrupt depletion of glutathione in cultured rat hepatocytes. Arch. Biochem. Biophys. 265: 311–20. Moelwyn-Hughes, E.A., and Owens, R., The Surface Tension, The Molecular Surface Energy and the Parachor of Toxic Compounds and of Certain Chlorides Used in Their Manufacture, Part XV of the Thermal Decomposition of the Secondary Alkylflurophosphonites, Sutton Oak Report 544, Sutton Oak, England, September 1941, UNCLASSIFIED Report. Munro NB, Watson AP, Ambrose KR, Griffin GD. Treating exposure to chemical warfare agents: implications for health care providers and community emergency planning. Environ Health Perspect 89:205-215 (1990). Murphy, M.L., Del Moro, A., Lacon, C. (1958). The comparative effects of five polyfunctional alkylating agents on the rat fetus, with additional notes on the chick embryo. Ann. NY Acad. Sci. 68: 762–82. NATO, NAVMED P-5059 NATO Handbook on the Medical Aspects of NBC Defensive Operations, NATO Headquarters, 1996, pp. 286 NDRC (National Defense Research Committee) (1944). Geiling, E.M.K., Cannan, R.K., Bloom, W. Toxicity of chemical warfare agents. NDRC-IMPR-9-4-1-17. June 1944. NDRC (National Defense Research Committee) (1945). The penetration of vesicant vapors into human skin. Rockefeller Inst. Med. Res. OSRD Report No. 4855, March 24, 1945. NRC (National Research Council). 1999. Review of the U.S. Armys Health Risk Assessments for Oral Exposure to Six Chemical-Warfare Agents. Committee on Toxicology, National Research Council, Washington, DC. NRC (National Research Council) (2003). Acute Exposure Guideline Levels for Selected Airborne Chemicals. Subcommittee on Chronic Reference Doses for Selected Chemical-Warfare Agents, Committee on Toxicology, Board on Environmental Studies and Toxicology, Commission on Life Sciences, National Research Council. National Academy Press, Washington, DC. Reed, C.I. 1918. The Minimum Concentration of Mustard Gas Effective for Man (Preliminary Report), Report No. 318. Pharmacology Research Section, American University. Reed, C.I. 1920. The Minimum Concentration of Dichlorethylsulfide (Mustard Gas) Effective for Man. J. Pharm. Exp. Therap. 15:77-80. Reed, C.I., E.F. Hopkins and C.F. Weyand. 1918. The Minimum Concentration of Mustard Gas Effective for Man (Final Report). Report No. 329, War Department, Medical Division, C.W.S., Pharmacology Research Section, American University Experiment Station, Washington, D.C. Papirmeister, B., A.J. Feister, S.I. Robinson and R.D. Ford. 1991. Medical Defense Against Mustard Gas: Toxic Mechanisms and Pharmacological Implications. CRC Press, Boca Raton, FL. 359 pp. Penski, E.C., Properties of Di-(2-Chloroethyl) Sulfide I. Vapor Pressure Data Review and Analysis, ERDEC-TR-043, USA Edgewood Research, Development and Engineering Reference-12 Center, Aberdeen Proving Ground, MD, April 1993, UNCLASSIFIED Report (ADA267059). Renshaw, B. (1947). Observations on the role of water in the susceptibility of human skin to injury by vesicant vapors. J. Invest. Dermatol. 9: 75–85. Roberts, J.J. and G.P. Warwick. 1963. Studies of the mode of the action of alkylating agents - VI. The metabolism of bis-ß-2-chloroethylsulphide (mustard gas) and related compounds. Biochem. Pharmacol. 12:1329-1334. Romano, J.A., Lukey, B.J., Salem, H. (2008). Chemical Warfare Agents: Chemistry, Pharmacology, Toxicology, and Therapeutics. CRC Press, Boca Raton, FL. Rosenblatt DH, Miller TA, Dacre JC, Muul I, Cogley DR. Problem Definition Studies on Potential Environmental Pollutants. II. Physical, Chemical, Toxicological, and Biological Properties of 16 Substances. Tech Rpt 7509; AD AD30428. Fort Detrick, MD:U.S. Army Medical Bioengineering Research and Development Laboratory, 1975. Rosenblatt DH, Small MJ, Kimmell TA, Anderson AW. Agent Decontamination Chemistry: Technical Report. U.S. Army Test and Evaluation Command (TECOM) Technical Support, Phase 1. Prepared for the Environmental Quality Office, U.S. Army Test and Evaluation Command, by Argonne National Laboratory, Washington, DC, 1995. Ruth, J.H. (1986). Odor thresholds and irritation levels of several chemical substances: a review. Am. Ind. Hyg. Assoc. J. 47: A-142–51. Samuel, J.B., et al., Physical Properties of Standard Agents, Candidate Agents, and Related Compounds at Several Temperatures (U), ARCSL-SP-83015, USA Armament Research and Development Command, Aberdeen Proving Ground, MD, June 1983, UNCLASSIFIED Report (ADC033491). Sasser L.B., R. A. Miller, D.R. Kalkwarf, et al. 1989a. Toxicology Studies on Lewisite and Sulfur Mustard Agents: Subchronic Toxicity of Sulfur Mustard (HD) in Rats. Final Report , PNL-6870, Pacific Northwest Laboratories, Richland, WA. Prepared for the U.S. Army Medical Research and Development Command, Fort Detrick, MD. Schier, J.G., Hoffman, R.S. (2005). Equipment preparedness for terrorism. In Medical Response to Terrorism (Keyes, D.C., Burstein, J.L., Schwartz, R.B., Swienton, R.E., eds), pp. 284–92. Lippincott Williams & Wilkins, Philadelphia, PA. Shakil, F.A., A. Kuramoto, M. Yamakido, et al. 1993. Cytogenetic abnormalities of hematopoietic tissue in retired workers of the Ohkunojima poison gas factory. Hiroshima J. Med. Sci. 42:159-165. Sidell, F.R. and C.G. Hurst. 1992. Clinical Considerations in Mustard Poisoning. In: Chemical Warfare Agents. A.M. Somani, ed., pp. 51-67, Academic Press, New York. Smith, M.G., Stone, W., Ren-Feng, G. et al. (2008). Vesicants and oxidative stress. In Chemical Warfare Agents, Chemistry, Pharmacology, Toxicology, and Therapeutics (J. Romano, Jr., CHAPTER 8 $ Mustards and Vesicants 107 B. Lukey, H. Salem, eds), pp. 293–312. CRC Press, Boca Raton, FL. Somani, S. M.; Babu, S. R. Toxicodynamics of Sulfur Mustard. Int. J. Clin. Pharm. Therap. Toxicol. 1989, 9, 419–435; Somani, S.M. 1992. Toxicokinetics and toxicodynamics of mustard. In: Chemical Warfare Agents. A.M. Somani, ed., pp. 13-50, Academic Press, New York. USACHPPM (US Army Center for Health Promotion and Preventive Medicine) (1996). Detailed and general facts about chemical agents – TG 218. USACHPPM, Aberdeen Proving Ground, MD. USACHPPM TG No. 218. October 1996. USACHPPM (US Army Center for Health Promotion and Preventive Medicine) (1999). Derivation of health-based environmental screening levels for chemical warfare agents: a technical evaluation. Aberdeen Proving Ground, MD. USACHPPM (US Army Center for Health Promotion and Preventive Medicine) (2000). Evaluation of airborne exposure limits for sulfur mustard: occupational and general population exposure criteria. Aberdeen Proving Ground, MD. Technical Report 47-EM-3767-00. USACHPPM (US Army Center for Health Promotion and Preventive Medicine) (2004). Acute toxicity estimation and operational risk management of chemical warfare agent exposures. USACHPPM Report No. 47-EM-5863-04. USACHPPM, Aberdeen Proving Ground, MD. May 2004. USACS, Potential Military Chemical/Biological Agents and Compounds, US Army Chemical School, 2001, pp. 130 Vijayaraghavan, R. 1997. Modifications of breathing pattern induced by inhaled sulphur mustard in mice. Arch. Toxicol. 71:157-164. Watson AP, Ambrose KR, Friffin GD, Leffingwell SS, Munro NB, and Waters LC, “Health Effects of Warfare Agent Exposure: Implications for Stockpile Disposal,” Environ Prof, 11, 1989, pp. 335–353. Watson AP, and Griffin GD, “Toxicity of Vesicant Agents Scheduled for Destruction by the Chemical Stockpile Disposal Program,” review, Environ Health Perspect, 98, 1992, pp. 259–280. Williams, A.H., “The Thermal Decomposition of 2:2’-Dichlorodiethyl Sulphide,” J. Chem. Soc., 1947. Wils, E.R.J., A.G. Hulst and J. Van Laar. 1988. Analysis of thiodiglycol in urine of victims of an alleged attack with mustard gas. Part. II. J. Anal. Toxicol. 12:15-19. Witten, Benjamin, The Search for Toxic Chemical Agents (U), EATR 4210, Edgewood Arsenal Research Laboratories, MD, November 1969, UNCLASSIFIED Report (AD507852). Yang, Y., Baker, J., and Ward, J. “Decontamination of chemical warfare agents”, Chem. Rev., 92, 1729-1743 (1992). Yang, Y., et al., “Characterization of HD Heels and the Degradation of HD in Ton Containers,” In Proceedings of the 1996 ERDEC Scientific Conference on Chemical and Biological Defense Research 19-22 November 1996, UNCLASSFIED Paper, ERDEC-SP-048, USA Edgewood Research, Development and Engineering Center, Aberdeen Proving Ground, MD, October 1997, UNCLASSFIED Report (ADA334105). Young, L., J.A. McCarter, M. Edson and E. Estok. 1944. Biochemical Experiments with Mustard Gas Prepared from Radioactive Sulfur. V. The Systemic Distribution of 35S at Different Times after Application of Radioactive Mustard Gas to the Skin of the Rat. University of Toronto, Report No. 17, C.P. 75. Zajtchuk R., Textbook of Military Medicine: Medical Aspects of Chemical and Biological Warfare, U.S. Department of Defense, 2000, pp. 721 |
||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||